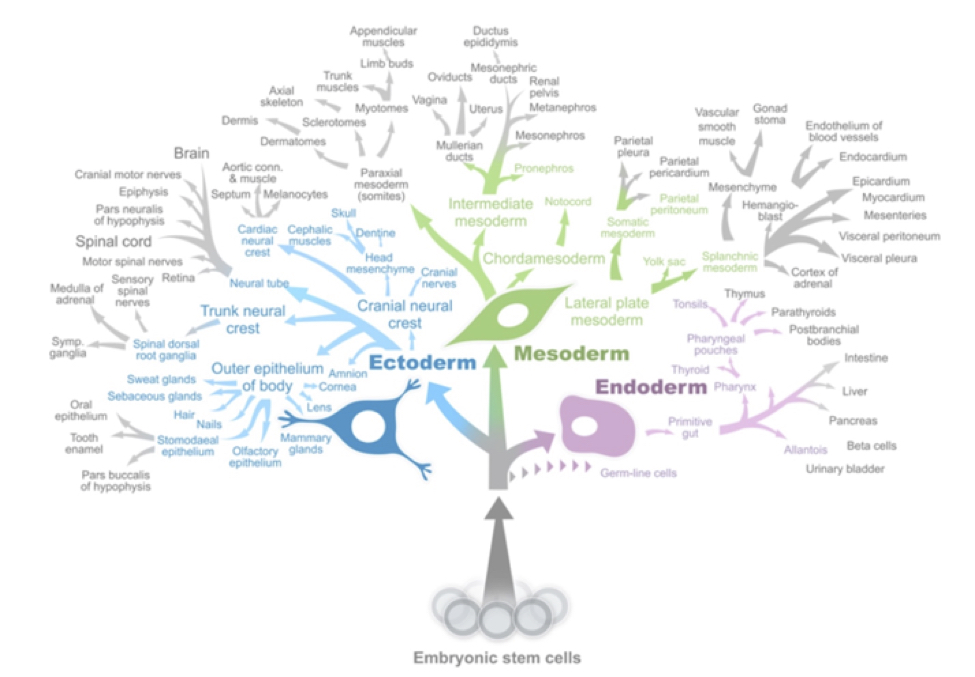
Pluripotent Stem Cells possess both telomerase-mediated replicative immortality and regenerative potential, and are capable of producing all human cell types
Pluripotent Stem Cells possess both telomerase-mediated replicative immortality and regenerative potential, and are capable of producing all human cell types
In the mid-1990s, AgeX’s CEO Dr. Michael West, organized a collaboration with Drs. James Thomson, John Gearhart and Roger Pedersen that led to the first isolation of Pluripotent Stem Cells (PSCs). In contrast to other types of cells, PSCs are unique by at least two important criteria. The first criterion relates to the ability of pluripotent cells to proliferate, or make more copies of themselves indefinitely, that is to say they are “immortal”. The second criterion relates to the ability of PSCs to differentiate into any of the hundreds of specialized cell types in the body. This replicative immortality of PSCs facilitates the industrial scale-up of product. We believe many of these cell types have potential for regenerating function in tissues damaged by degenerative diseases when transplanted into the body.
Unlike immortal PSCs, adult stem cells typically have severely reduced scale-up potential because they are mortal. In addition, adult stem cells have passed the Weismann Barrier and are hence limited in their ability to regenerate normal tissue when transplanted in vivo. Therefore, we believe that PSC-based cellular therapeutics have significant competitive advantages over cell-based therapeutics being developed by the many adult stem cell companies.
PureStem® Technology
Regulatory approval of cell and tissue-based products requires high standards of quality control. In the case of stem cell-derived products, there is a high standard for insuring the known identity, purity, and reproducibility of the cells to be administered. Pluripotent Stem Cells (PSCs) such as Embryonic Stem (ES) cells and induced Pluripotent Stem (iPS) cells provide certain advantages over adult-derived cells when used in the manufacture of cell-based therapeutics for the treatment of age-related disease. These advantages include:
- The replicative immortality of PSCs which facilitates the indefinite scale-up of PSC master cell banks for the manufacture of uniform product, as well as an immortal substrate for targeted genetic modifications.
- Since most PSCs maintain long and stable telomere lengths, the replicative capacity of derived differentiated cell types is typically longer (and younger) than adult or even fetal-derived cells.
- Using PureStem® technology, it is possible to clonally expand hundreds of purified, identified, and reproducibly scalable cell types that retain regenerative potential (i.e. they have not passed the regeneration limit).
PureStem® technology is based on the observation that early in our development, tissues in the human body are naturally comprised of highly proliferative cells with relatively long cell lifespans. Therefore, it is possible to generate single cell-derived (clonal) lineages of these cells in the laboratory, a process not easily achievable with cells derived from adult tissues which commonly senesce during clonal expansion.
In addition, adult tissues largely contain differentiated cells often with limited or no capacity of replication in vitro. As a result, the clonal expansion of our proprietary PureStem® lines allows not only a novel and more facile point of scalability, but also generates populations of cells that are multipotent instead of pluripotent, and hence markedly easier to define identity, purity, and potency.
We have studied the fate of over 200 diverse PureStem® cell lines in thousands of differentiation conditions. This was accomplished by thawing individual cryopreserved PureStem® cell lines, culturing them in the laboratory, and then exposing the cells to factors that differentiate cells such as protein, growth and differentiation factors, hormones, and/or small molecules implicated in causing cells to change from one type of cell into another (differentiation). Using individual cells from over 200 diverse PureStem® cell lines previously isolated and cryopreserved, we treated the diverse cells with thousands of differentiation conditions, prepared RNA, and determined the gene expression pattern of the cells using gene expression microarrays. These experiments have shown that the PureStem® cell lines display site-specific markers that identify not only the type of cells, but also where in the body the cells would normally reside. Therefore, in the example of cartilage cells, it was possible to produce diverse types of cartilage in this manner.
Applications outside of orthopedics, medical aesthetics, and certain ophthalmological applications are licensed from BioTime to AgeX. AgeX has chosen two applications for its initial product development based on unmet medical need along with other factors: First, Brown Adipose Tissue (BAT) cells for the treatment of Type II diabetes and second, vascular endothelial progenitors for the treatment of age-related ischemic disease such as that leading to myocardial ischemia and infarction.
induced Tissue Regeneration (iTR™)
The premise behind iTR™ is that aging, and in turn, degenerative diseases associated with old age, are due to the loss of two characteristics that are present in the reproductive cells of our body, but are progressively lost in most of the other cells as a result of developmental aging. These two characteristics are:
- Replicative immortality of cells
- Regenerative capacity for most of the cell types in the body
Historically speaking, the scientists in our team have consistently led not only in the discovery of these facts, but also by inventing novel therapeutic strategies to reverse cellular and developmental aging. These include the role of telomerase in aging and cancer, pluripotent stem cells (that like reproductive cells have full replicative immortality and regenerative potential) and the reprogramming of the aging of cells by somatic cell nuclear transfer (SCNT) or defined genes.
Since 2010, when our scientists (then at BioTime, Inc.) demonstrated the reversal of the developmental aging of human cells using transcriptional reprogramming, our research has focused on developing a means of applying the technology to the aging human body. In 2017, our scientists published data regarding certain genes that were turned off or turned on at the same time regenerative potential was lost.
Since 2017, additional patents were filed in the U.S. and elsewhere on additional technologies relating to these discoveries (now 12 patent families in total). One example of these newer technologies are methods to precisely regulate the reprogramming of aging on a cellular level.

An example of a Developmentally-Regulated iTR (DR-iTR) vector using Adeno-Associated Virus (AAV) to precisely control the reprogramming of target cells.
The technology is designated “Developmentally-Regulated Induced Tissue Regeneration” (DR-iTR). DR-iTR uses the regulatory promoter DNA of genes that are turned off or on at just the target developmental age we desire to reach to turn the age reversal genes on or off at just the right times.
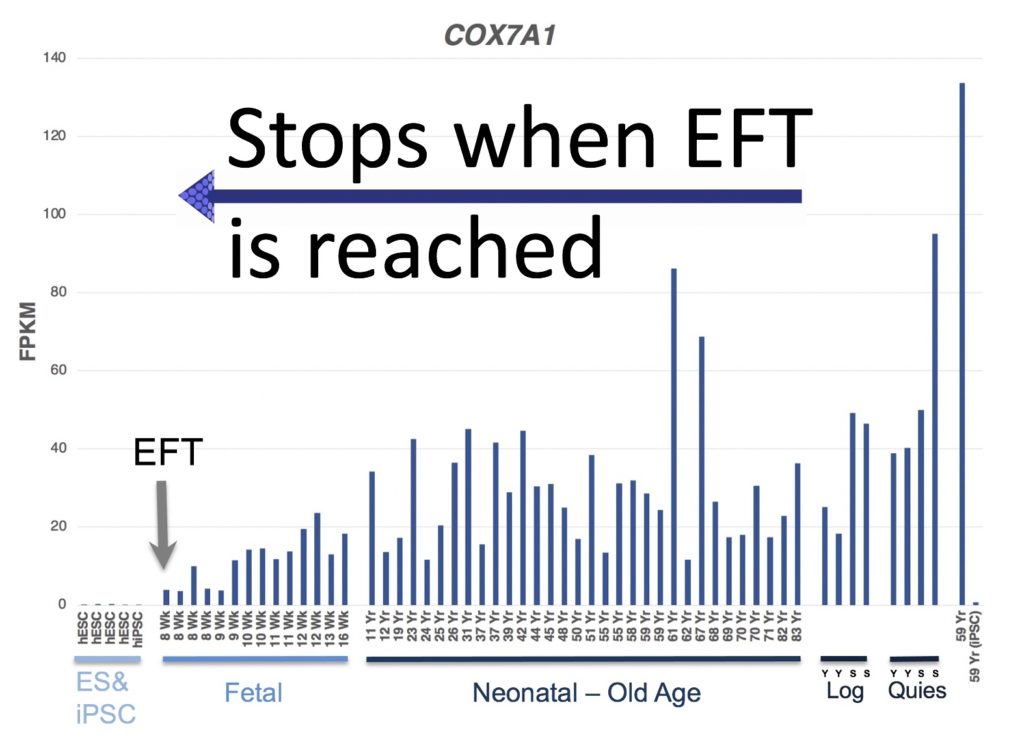
The gene COX7A1 turns on just as cells (in this case skin cells) lose regenerative potential. Therefore, DR-iTR genes would be expressed in non-regenerative cells, but would then turn off once the target age (EFT) is reached.
What makes iTR so interesting is that the mechanisms that regulate this profound regenerative potential are common to most of the cell and tissue types in the body. Therefore, while we plan to initially develop the product for dermatological applications (a product we designate “RENELON”), it may prove useful in treating chronic degenerative conditions in diverse fields of medicine including cardiology, neurology, and even in cancer.
Our scientists have discovered that, like telomerase which has a duel role in aging and cancer, the molecular pathways regulating regeneration are a previously-unrecognized hallmark of most cancer types (pan-cancer).
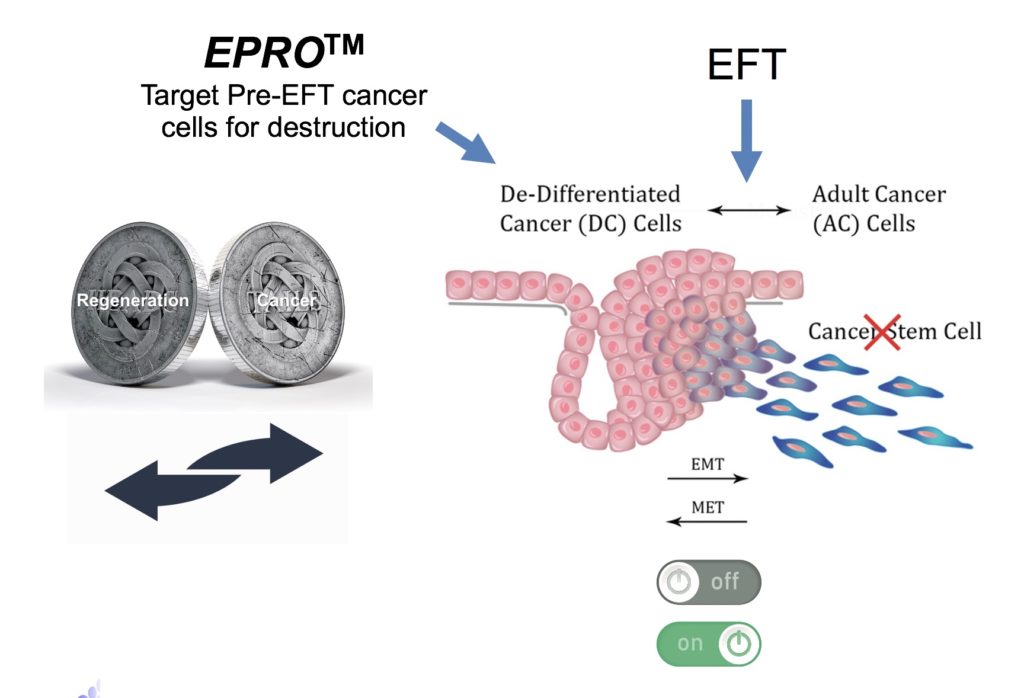
The flip side of the coin of the molecular pathways discovered by AgeX scientists is that cancer cells can reside in either the regenerative state (pre-EFT) or a non-regenerative state (post-EFT). Recognizing this has led our scientists to file patents on novel therapeutic and diagnostic strategies for diverse types of cancer.
Cancer cells are frequently in a primitive regenerative state (pre-EFT). Our scientists have discovered that what are commonly called “cancer stem cells” are actually post-EFT (adult-like) cancer cells, not more undifferentiated as is commonly believed. This discovery led our scientists to invent new oncolytic strategies to destroy pre-EFT cancer cells using gene sequences common to most cancer types (pan-cancer). This product planned for development is designated EPRO™
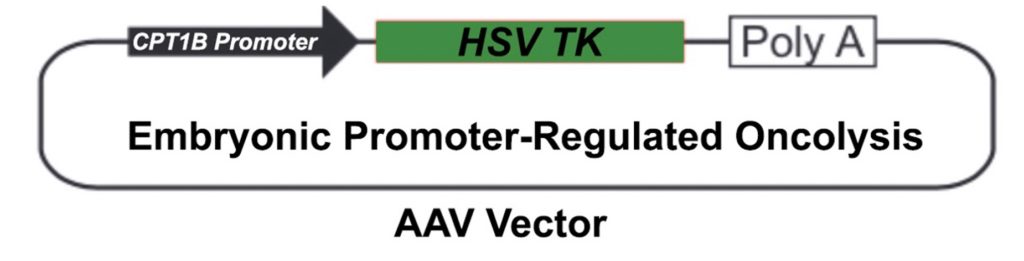
EPRO uses promoters like that for the gene CPT1B, which turns off in adult cells, but is expressed in diverse types of pre-EFT regenerative cells and diverse cancer types. Therefore, potentially toxic genes like HSV TK can potentially be used to target cancer cells for selective destruction.
Furthermore, AgeX scientists have discovered a family of cell surface proteins that are expressed in pre-EFT cells and diverse cancer cells, but not in most normal adult cell types. One family of such cell surface proteins is the alpha clustered protocadherins.

Cell surface proteins, such as certain members of the clustered protocadherin family (genes of the alpha cluster shown here) provide useful targets for cancer therapy since they are abundantly expressed in pre-EFT regenerative cells such as the diverse embryonic progenitors (EPs) shown, but not expressed in most adult cell types with the exception of brain cells. They are, however, expressed in diverse cancer cell types in the pre-EFT state.
This discovery potentially allows therapeutics, such as EPRO, or other technologies such as CAR T-Cell therapies to selectively target cancer cells.
In summary, AgeX’s patent estate in iTR includes some of the earliest filed patents and patent applications in the field (priority dates ranging from 2013 to 2021), and teach applications in diverse age-related degenerative diseases as well as diverse types of cancer.
UniverCyte™
UniverCyte™ is a proprietary technology to modify cells such that they can potentially be transplanted into all patients “off-the-shelf”, that is, without the normal need to match cells to the patient. The process uses a molecule called HLA-G. In nature, HLA-G’s primary function is to prevent immune rejection of a baby by the mother. Therefore, we believe our HLA-G modified PureStem® cell therapies will utilize a natural pathway of preventing transplant rejection and hence may have certain benefits over current immunosuppressive strategies.
